Quantum mechanics
...is our most fundamental theory of how the world works.
I'll be following along with Richard Feynmann's Lectures on Physics, volume 3.
Experiments with bullets
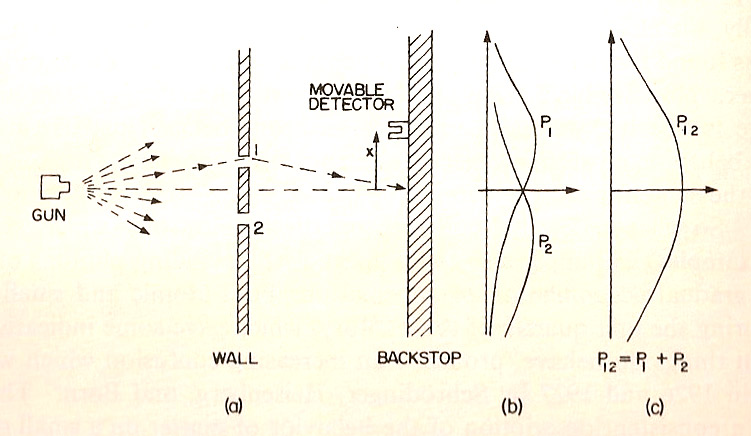
- Detector counts bullets
- $P_1$ is the probability (as a function of position) that a bullet will land in the detector when only slit 1 is open.
- $P_2$ is the probability...when only slit 2 is open.
- $P_{12}$ is the probability that a bullet will land in the detector when both slits 1 and 2 are open.
- $P_{12}=P_1+P_2$.
Experiments with waves
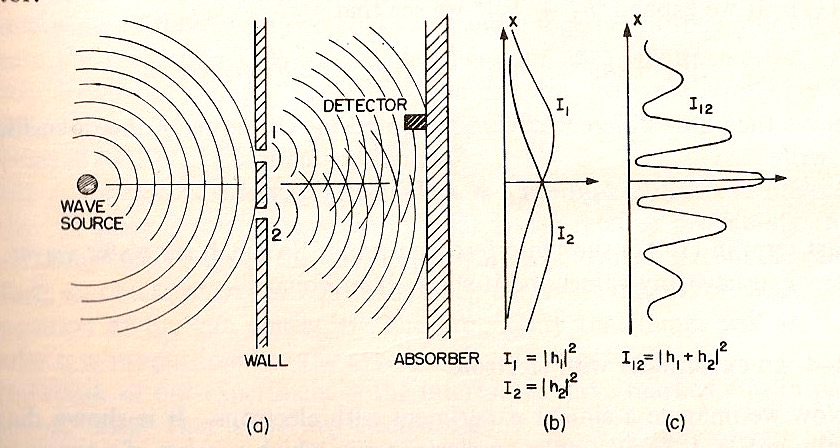
- The detector is sensitive to the intensity (energy) of the wave passing by.
- A wave can be written as a function of position and time: $$h(x,t)=h_0\sin(\omega t-k x)$$ where $h_0$ is the amplitude.
- The intensity (or energy) of wave at a particular position is the time average of the amplitude squared: $$I=\int_0^{\text{1 period}}|h_0\sin(\omega t-k x)|^2\,dt\propto h_0^2.$$
- Wave interference happens before the detector: There can be constructive or destructive interference.
Experiments with electrons
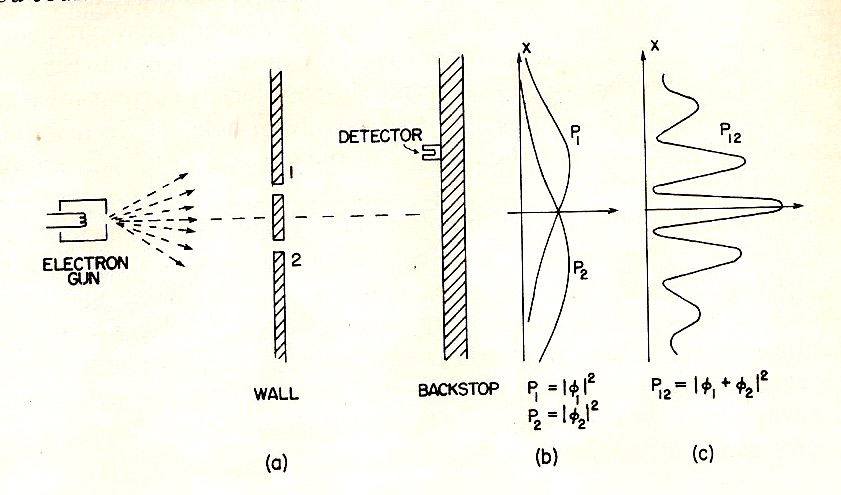
- Individual electrons are detected.
- But an interference pattern occurs.
- $\Rightarrow$ as if waves ("probability waves") were interfering, and the wave "height" determines the probability of detection.
A different experiment with electrons
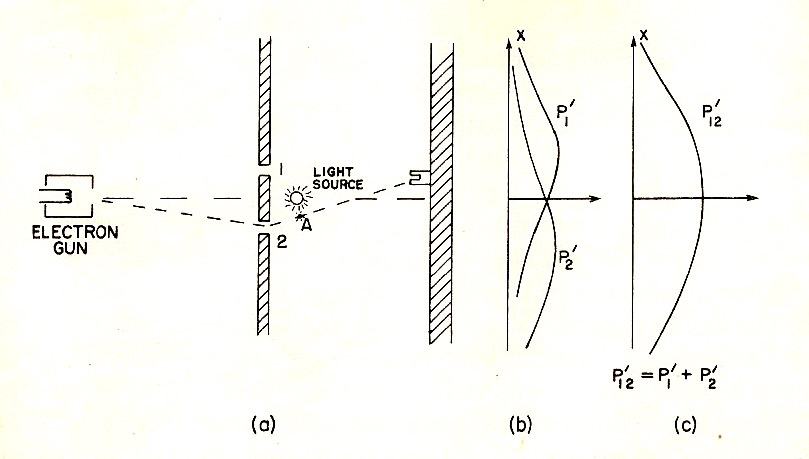
Shine a light. Light "bounces off" an electron, and you can *see*
which slit the electron is closest to.
- When you do this, the interference pattern is destroyed. You observe the "bullet" batter instead.
Another experiment: Turn down the intensity of the light:
- Sometimes you will see a flash and count an electron.
- Sometimes you will count an electron and *not* see a flash.
- Keep track of which counts came from electrons you could *see*: They form the bullet pattern.
- Keep track of which counts came from electrons you couldn't see: They form the interference pattern.
Do only small electrons and photons have "wave nature"?
Juffman et al demonstrate double slit interference demonstrated with massive molecules. [Movie]
Louis de Broglie hypothesized that everything has an effective "de Broglie" wavelength of $$\lambda = \frac hp.$$