Seeing heat?
We can "see" objects glowing when they get above about 1000 F.
Some snakes can sense much lower temperatures with their 'pit organs'.
Somehow, this is related to the Greenhouse Effect!
What is heat??
 Atomically, it's related to the average speed of molecules. The higher the temperature, the faster molecules are moving. (Here's a more elaborate simulation).
Atomically, it's related to the average speed of molecules. The higher the temperature, the faster molecules are moving. (Here's a more elaborate simulation).
Losing heat
When a hot object cools down we say it loses energy. It's thermal energy is lower the colder it gets.
The most familiar way to lose thermal energy is by contact with something colder.
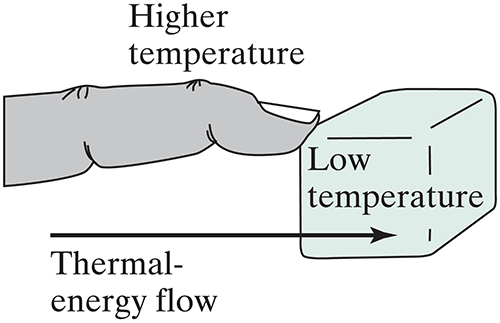
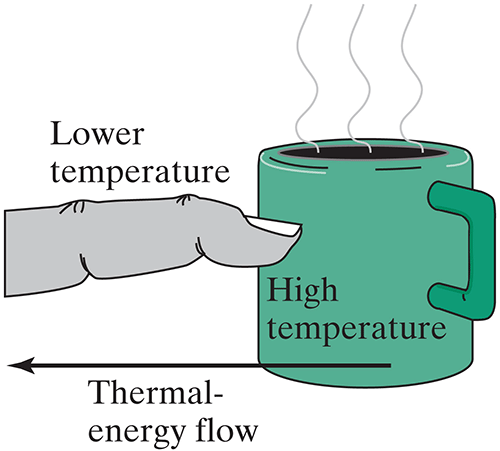
 But there is a second way that hot objects can lose energy: What form of energy is coming from the hot kiln that you can see?
But there is a second way that hot objects can lose energy: What form of energy is coming from the hot kiln that you can see?
Light = Electro-magnetic waves
Whenever you shake (accelerate) some charged particles you generate electro-magnetic waves.
Electro-magnetic waves are just another name for light. The faster the atoms are moving, the more frequently they bump into each other, and the average frequency of light increases when the temperature increases.
Frequency and wavelength
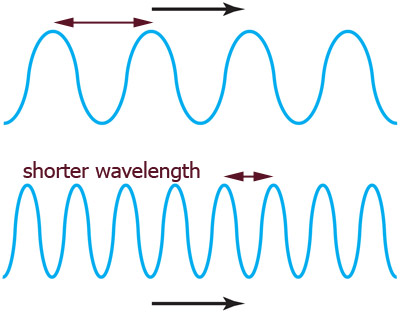 Frequency =
# of waves passing a point in a time interval - [1/s] or [(cycles)/sec] or
[Herz] =[Hz]
Frequency =
# of waves passing a point in a time interval - [1/s] or [(cycles)/sec] or
[Herz] =[Hz]
What happens to the wavelength if you shake a slinky with a higher frequency??
It turns out that frequency$\equiv f$ and wavelength$\equiv \lambda$ are related to the speed$\equiv c$ of a wave like this: $$f\lambda=c$$
If all other things (amplitude and speed) are equal do you think a higher frequency wave has more or less energy than a low frequency wave??
Imagine a boat tied to a dock being tossed up and down by these two waves. Which wave is doing more work against gravity by lifting the boat more often??
Going back to heat, do you think charged atoms are shaking slower or faster when they are heated to a higher temperature?
Electromagnetic spectrum
Higher temperature = faster shaking = higher frequency waves.
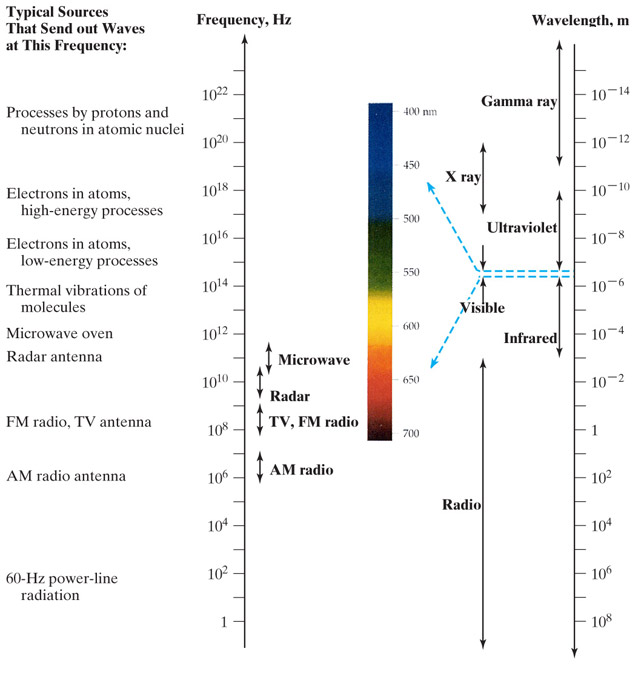
Blackbody radiation
Our sun has a surface temperature of ~5400 C~5700 K.
A Tungsten lightbulb filament has a melting temperature of ~3700 K.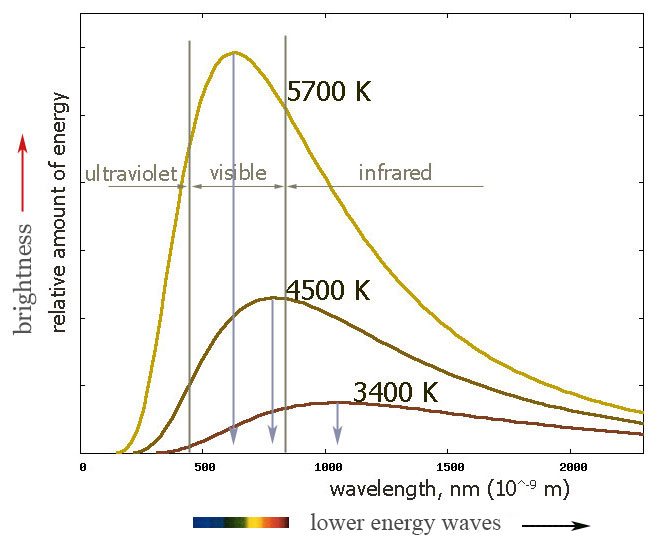
The temperature of a glowing object is closely related to its apparent color.
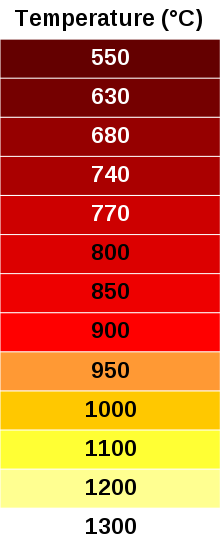 We see objects glowing visibly, once they get above about 1000 F.
We see objects glowing visibly, once they get above about 1000 F.
Do we glow too?
Gee wouldn't it be nice if we had some way of sensing lower frequency light?
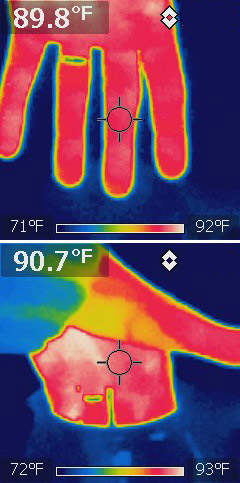
Special sensors can sense IR (infra-red) region of the spectrum, and then transpose it up into the visible range for us to see. This is the basis of an IR camera. Just like the visible scale, the camera can estimate the temperature from the wavelength of light given off:
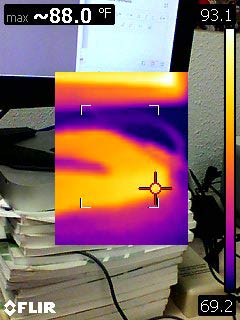
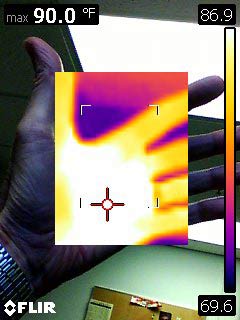
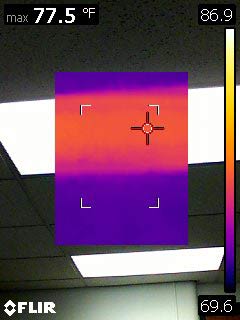


Interesting: The incandescent bulb is much hotter than the fluorescent light in my ceiling... But much cooler than 1000 F? What happens when we turn off the light bulb?
You can also use this technology to look for heat leaks: Here are two sets of windows on a 6th St house on the same cold night:
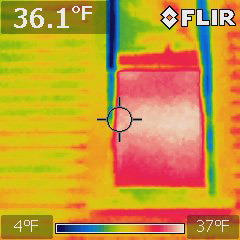 Older window |
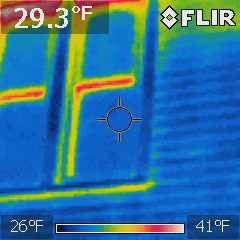 Replacement, highly insulated window |
|---|
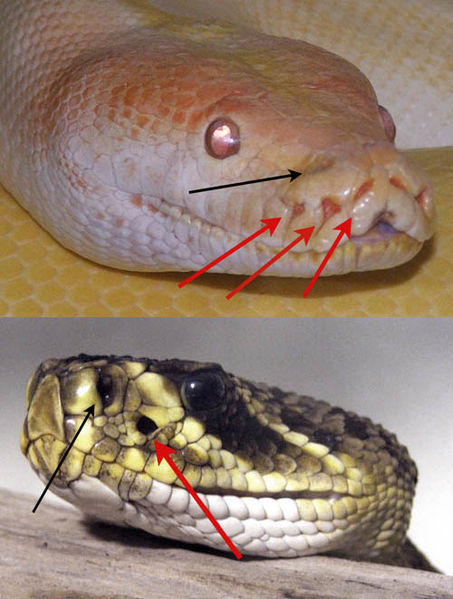
Some snakes have "pit organs" that can sense IR radiation.
Transparency depends...
 Whether an object:
Whether an object:
- allows light to pass through ("transparent"), or
- does not allow light to pass through ("opaque" or "absorbs light")
...apparently depends on the wavelength of the light.
We can "see" the sun very easily. Gases in the atmosphere like Nitrogen, oxygen, Carbon dioxide, water vapor (though not condensed water vapor = clouds), methane, hydrogen, etc are transparent to visible light.
But some gases in the atmosphere are less transparent to infrared light. These are the "greenhouse gases":
- Carbon dioxide,
- water vapor,
- methane,
- $NO_x$
- CFC's (refrigerants from A/C systems)
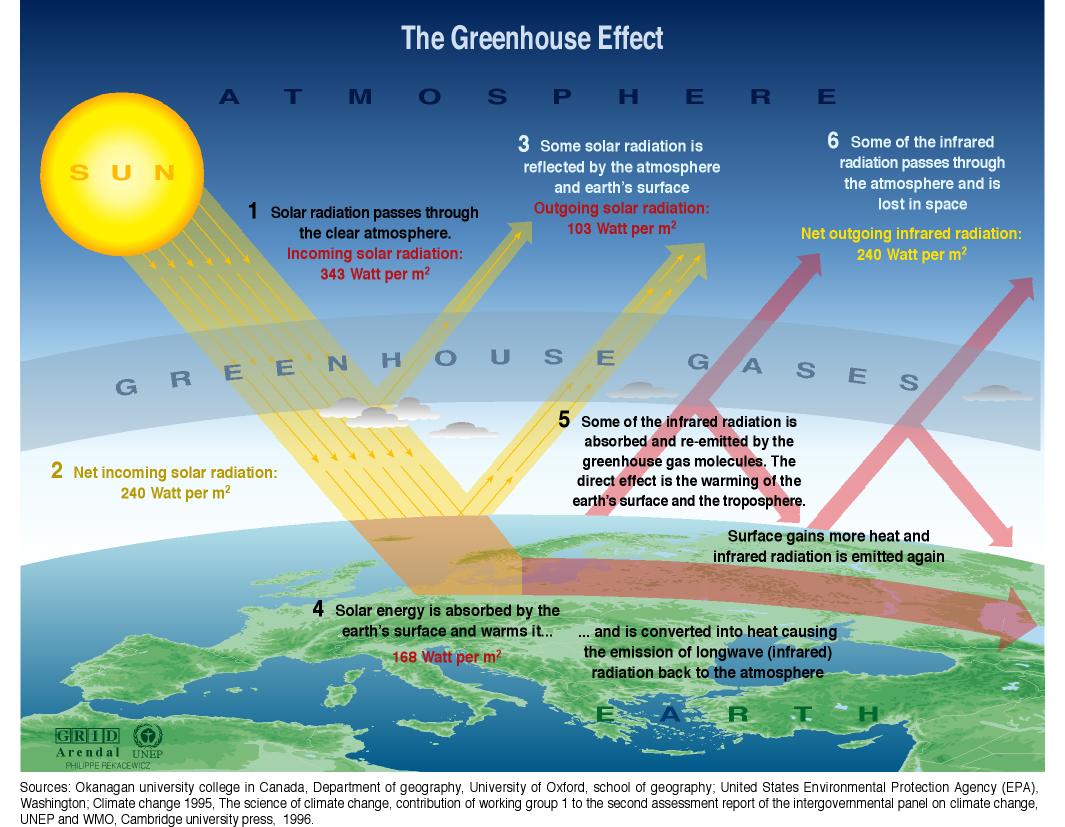
The Greenhouse Effect
of Earth's atmosphere
- Visible light from the sun is transmitted through the atmosphere (including the GHGs):
- The visible light strikes Earth's surface where it is absorbed by land/roads/trees (friction): These objects heat up.
- Hot objects give off infrared (IR) radiation.
- But GHGs reflect and absorb IR radiation; They are not as transparent to IR light. [ IR=infrared: Light with a wavelength much longer than visible red light. ]
So some energy from the sun remains trapped in the atmosphere, which heats up the atmosphere.
 Substitute "car windows" for "GHGs" in the description above, and you have an explanation for why the inside of your car on a sunny day is hotter than
the surroundings.
Substitute "car windows" for "GHGs" in the description above, and you have an explanation for why the inside of your car on a sunny day is hotter than
the surroundings.
Thanks to the natural greenhouse effect the surface of earth has an average temperature of about 14 C (~60 F).
Without this, (Oxygen and nitrogen but no GHGs) it is estimated the average temperature would drop to -18 C (~ 0 F).
Image credits
Insolation map: NREL
Phil G, D Searls, Flickr user Zen, Wikipedia user Kendrick7 and S.n.