Building a nuclear bomb
How to get 'critical'
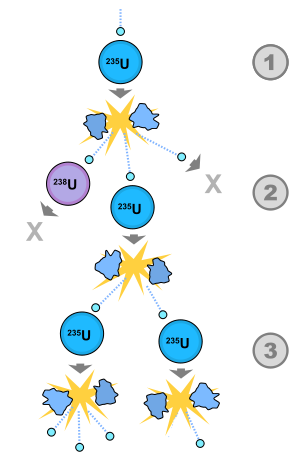 It's harder than you think to start a chain reaction:
It's harder than you think to start a chain reaction:
- Higher purity -> technically difficult, but removing U-238 means less (fast) neutron absorption.
 If you had two chunks of the same weight of pure U-235, which shape would be more likely to start a chain reaction?
If you had two chunks of the same weight of pure U-235, which shape would be more likely to start a chain reaction?
In which shape would an emitted neutron be more likely to run into another U-235 nucleus?
Critical mass
The critical mass is the minimum amount needed to keep a chain reaction going.

 Pure U-235 - 50 kg sphere
Pure U-235 - 50 kg sphere
Pure Pu-239 - 10 kg
A-bomb
style="text-align: right"> Bring 2 pieces that are not critical together into one that *is* critical.
Bring 2 pieces that are not critical together into one that *is* critical.
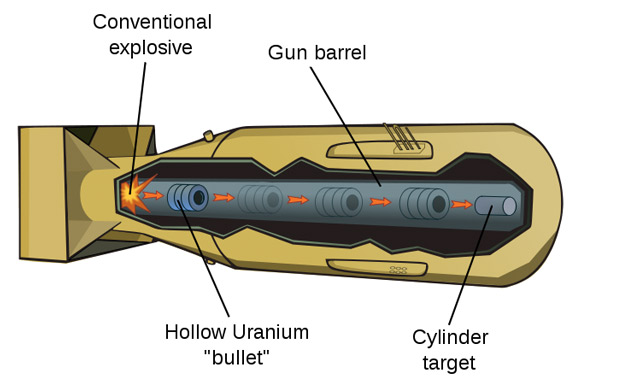
But where will the neutrons needed to *start* the chain reaction come from???
For 10 kg of Uranium-235, there is on average 0.1 neutrons / sec from spontaneous fissions, or one spontaneous fission each 10 sec.
It takes about 0.001 s ($10^{-3}$ seconds) for the two chunks to slide into place.
Plutonium
"Weapons-grade" plutonium-239 still has about 5% plutonium-240, with a spontaneous fission rate (0.05 kg out of 10kg) of 46,000 neutrons / sec from spontaneous fissions, or 1 fission each $2.2\times 10^{-5}$ sec.
What will happen if it takes $1\times 10^{-3}$ sec to make the critical mass?
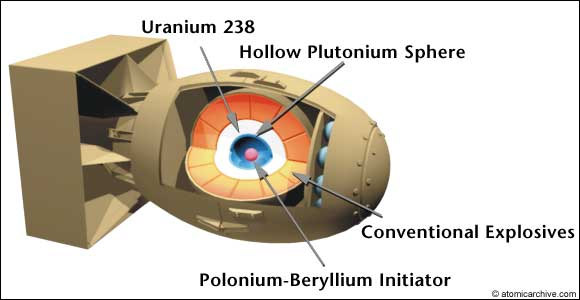
So, you can't build a plutonium bomb the same way: the plutonium would fizz as the chunks get close, but not explode.
To make a plutonium bomb, you have to assemble the critical mass much faster: within a micro-second ($10^{-6}$ seconds).
This is done by packing explosives around a sub-critical mass of Pu, and setting them off to compress (implode) the plutonium.
The Manhattan project
1939 - Einstein lends his name to a letter, written by Szilard, warning Roosevelt of the potential for a new kind of weapon based on chain-reaction fission in Uranium.
1941 - Japan attacks Pearl Harbor.
1942 - The Manhattan Engineering District turns earnestly into a bomb-making project. Two tracks will be pursued:
- a ${}^{235}$U bomb, and
- a Plutonium bomb.
${}^{235}$U bomb
One ${}^{235}U$ nucleus is chemically identical to the 140 ${}^{238}$U that surround it. How to separate out the fissionable ${}^{235}$U?
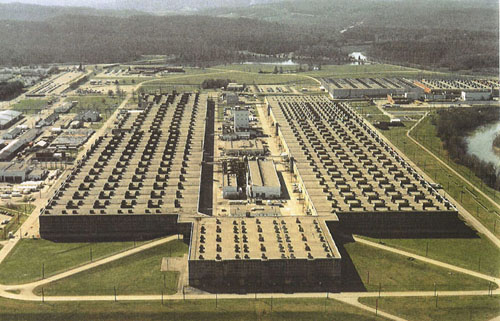 A huge gas diffusion plant was built at Oak Ridge, TN, that would eventually
consume power at a greater rate than all of New York City, and deliver ~50
kg of ${}^{235}$U by the end of the war.
A huge gas diffusion plant was built at Oak Ridge, TN, that would eventually
consume power at a greater rate than all of New York City, and deliver ~50
kg of ${}^{235}$U by the end of the war.
Gas centrifuges
 More recently
the U.S., and countries like Pakistan have used gas centrifuges to enrich
the Uranium.
More recently
the U.S., and countries like Pakistan have used gas centrifuges to enrich
the Uranium.
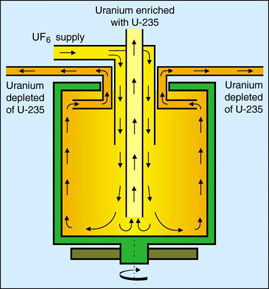 Uranium
is made into a gas -- $UF_6$-- and spun rapidly in such a cylinder. The heaver
isotopes settle towards the outside of the container.
Uranium
is made into a gas -- $UF_6$-- and spun rapidly in such a cylinder. The heaver
isotopes settle towards the outside of the container.
How to... make Plutonium
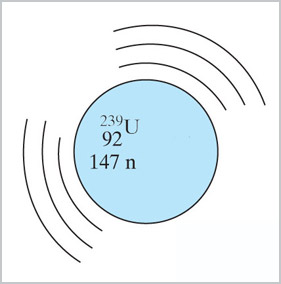
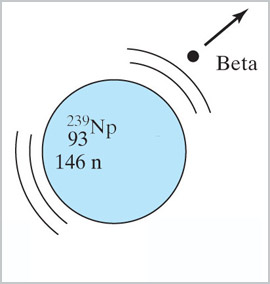
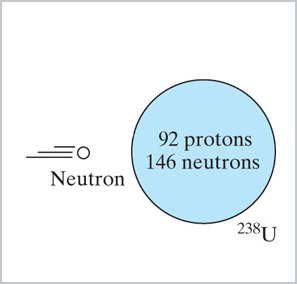 When ${}^{238}$U is bombarded with neutrons in a nuclear reactor, it does not
fission and give off more neutrons, but instead...
When ${}^{238}$U is bombarded with neutrons in a nuclear reactor, it does not
fission and give off more neutrons, but instead...



Plutonium bomb
 Plutonium: also more than 1 excess neutron per fission.
Plutonium: also more than 1 excess neutron per fission.
Can be chemically separated from U.
Technically more difficult to go critical.
A reactor at Hanford, WA was constructed to generate Plutonium.
Image credits
Laurent Bourneuf, German History in Documents and Images, Fission chain reaction, nukeworker.com, DOE digital archive, Hanford, Baysmom, Doistrakh, Dake, Atomic Archive, Joachim Reinhardt, Atomic Archive