Atoms / molecules
...and a bit about chemical energy.
Greek origins
[image: The young Rembrandt as Democritus the laughing philosopher]
 Leucippus
and his student Democritus (5th century BC) considered the possibility of
dividing a chunk of gold once, twice, many times and asked...
Leucippus
and his student Democritus (5th century BC) considered the possibility of
dividing a chunk of gold once, twice, many times and asked...
How long can this go on?
Either:
- You could keep dividing things forever, or else,
- you reach some smallest, indivisible entity, which cannot be divided further.
Write for 3 minutes... Which option do you like better? Why?
Democritus and Leucipus found 1.) distasteful, so chose to believe that there are "smallest things" (which they called "atoms").
They concluded that:
All matter is made of "atoms".
What observations or experiments did Democritus and Leucipus call upon to support their conclusion?
Elements, but not yet atoms
Before 1800 natural philosophers realized that...
- Many substances could be decomposed into other substances,
- A handful of substances could not further decomposed into other substances. They called these the elements
Modern example of "decomposition"
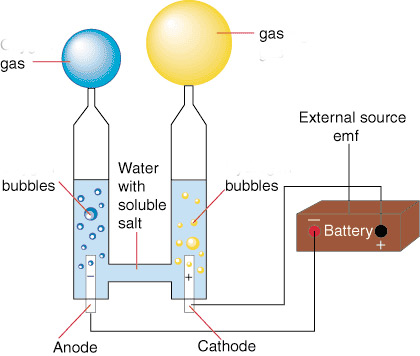 electrolysis of water
electrolysis of water
- Hook a battery to the circuit pictured, and electricity flows.
- At each of the platinum electrodes bubbles form.
- But at one of them, exactly twice as much gas (by volume) accumulates as at the other one.
It seems like the substances in the blue and yellow baloon are different: (They are not actually colored blue and yellow.)
- Purified water boils at 100${}^o$C, turning into a gas. We could also say that pure water vapor condenses into a liquid at 100${}^o$C as you cool it from higher temperatures.
- But the gas that accumulates in the blue balloon doesn't condense until cooled to -183${}^oC$.
- The gas that accumulates in the yellow balloon must be cooled to -253${}^o$C before it will condense.
The gas in the blue balloon we nowadays call oxygen, and the gass in the yellow balloon is hydrogen. Twice as much hydrogen accumulates as oxygen. Any idea why?
John Dalton
...was fascinated with the observation that, on decomposition, the resulting substances occurred in simple integer ratios.
 In the early 1800s John Dalton (a Quaker) noticed that one kind of tin oxide could be decomposed into 13.5 grams of oxygen for every 100 g of tin. Another kind could be decomposed into 27 grams of oxygen for every 100 g of tin.
In the early 1800s John Dalton (a Quaker) noticed that one kind of tin oxide could be decomposed into 13.5 grams of oxygen for every 100 g of tin. Another kind could be decomposed into 27 grams of oxygen for every 100 g of tin.
A simple integer ratio of 2:1 of the amounts of oxygen that went with 100 g of tin (Sb). Is this evidence of atoms??
John Dalton wrote:
Therefore we may conclude that the ultimate particles of all homogeneous bodies are perfectly alike in weight, figure, etc. In other words, every particle of water is like every other particle of water; every particle of hydrogen is like every other particle of hydrogen, etc.
Nowadays we distinguish a couple different kinds of "smallest particles".
- Atom - the smallest particle which has the chemical characteristics of a particular element. Examples: An argon (Ar) atom, carbon (C).
- Molecule - A long-lived particle consisting of 2 or more atoms that are chemically bound to each other. Examples: $H_2$, $H_2O$.
- Compound - a substance consisting of molecules that contain more than one kind of atom. Examples: $CH_4$, $H_2O$, but *not* $H_2$.
- A pure substance - contains only one kind of molecule or one kind of atom.
- A mixture - A substance containing more than one kind of molecule / atom.
We now know the chemical formulae for the two tin oxides (two different compounds) that Dalton considered as SbO and SbO${}_2$.
P.S.
Something else was happening with our mini-electrolysis cell in the course of passing it around. The liquid went from clear to yellowish:

It turns out that if your electrodes are rusty, hooking up the electrolysis cell will also "undo" the rusting process, changing your $Fe_2O_3$ (rust) back into $Fe$ but shedding some rust flakes into the water. This is a practical way to remove rust. The videos usually warn you that you'll also be producing hydrogen and oxygen while you do this!
