Atoms! / Chemical energy / Chemical reactions

"Releasing the chemical energy" in plants!
Elements, but not yet atoms
Before 1800 "natural philosophers" realized that...
- Many substances could be decomposed into other substances,
- A handful of substances could not be further decomposed into other substances. They called these the elements.
Decomposition demonstration
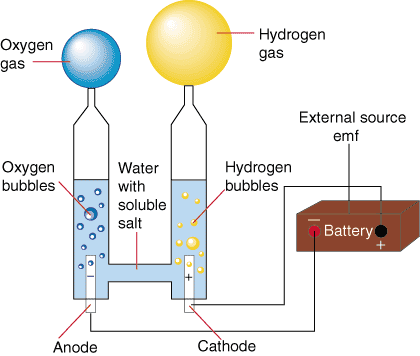 electrolysis of water
electrolysis of water
- Hook a battery to the circuit pictured, and electricity flows.
- At each of the platinum electrodes bubbles form.
- But at one of them, exactly twice as much gas (by volume) accumulates as at the other one.
Are they the same gas??
It seems like the substances in the blue and yellow baloon are different: (They are not actually colored blue and yellow.)
- Purified water boils at 100${}^o$C, turning into a gas. We could also say that pure water vapor condenses into a liquid at 100${}^o$C as you cool it from higher temperatures.
- But the gas that accumulates in the blue balloon doesn't condense until cooled to -183${}^oC$.
- The gas that accumulates in the yellow balloon must be cooled to -253${}^o$C before it will condense.
The gas in the blue balloon we nowadays call oxygen, and the gas in the yellow balloon is hydrogen. Twice as much hydrogen accumulates as oxygen. Any idea why?
John Dalton
...was fascinated with the observation that, on decomposition, the resulting substances occurred in simple integer ratios.
 In the early 1800s John Dalton (a Quaker) noticed that one kind of tin oxide could be decomposed into 13.5 grams of oxygen for every 100 g of tin. Another kind could be decomposed into 27 grams of oxygen for every 100 g of tin.
In the early 1800s John Dalton (a Quaker) noticed that one kind of tin oxide could be decomposed into 13.5 grams of oxygen for every 100 g of tin. Another kind could be decomposed into 27 grams of oxygen for every 100 g of tin.
A simple integer ratio of 2:1 of the amounts of oxygen that went with 100 g of tin (Sb). Is this evidence of atoms??
John Dalton wrote:
Therefore we may conclude that the ultimate particles of all homogeneous bodies are perfectly alike in weight, figure, etc. In other words, every particle of water is like every other particle of water; every particle of hydrogen is like every other particle of hydrogen, etc.
Nowadays we distinguish a couple different kinds of "particles".
- Atom - the smallest particle which has the chemical characteristics of a particular element. Examples: An argon (Ar) atom, carbon (C).
- Molecule - A long-lived particle consisting of 2 or more atoms that are chemically bound to each other. Examples: $H_2$, $H_2O$.
- Compound - a substance consisting of molecules that contain more than one kind of atom. Examples: $CH_4$, $H_2O$, but *not* $H_2$.
- A pure substance - contains only one kind of molecule or one kind of atom.
- A mixture - A substance containing more than one kind of molecule / atom.
We now know the chemical formulae for the two tin oxides (two different compounds) that Dalton considered as SbO and SbO${}_2$.
Back to electrolysis
We describe this situation with a chemical reaction equation: $$2H_2O_{\text{(l)}} \rightarrow 2H_\text{2 (g)} + O_\text{2 (g)}$$
The hydrogen ($H_2$) and oxygen ($O_2$) gas that appear cannot be decomposed further into other substances, so we say that hydrogen and oxygen are "elements".
Chemical balance
No atoms were harmed in this chemical reaction! The molecules that they make up were broken up and rearranged. No atoms are ever destroyed in any chemical reaction! That is:
Left side of the reaction: $2 H_2O$
2 molecules of $H_2O$. Each molecule has 2 atoms of H and 1 atom of O.
Total 4 atoms of hydrogen and 2 atoms of oxygen.
Right side of the reaction: $2 H_2 + O_2$
2 molecules of $H_2$ contain 4 atoms of H. 1 molecule of $O_2$ contains 2 atoms of oxygen.
Total 4 atoms of hydrogen and 2 atoms of oxygen.
Same number of each kind of atom on the left and on the right!
Balancing chemical reactions
Often you are in the situation of know what chemicals you start with (the "reactants"), and what chemicals you end up with (the "products"). To balance a chemical reaction you must figure out how many of each kind of molecule you need to make the number of each kind of atom come out the same on both sides.
Balance these chemical reactions (they are all "combustion" reactions, in which something burns by combining with oxygen)
Typically you will:
- Start by counting up the $C$ and $H$ atoms in the most complicated molecule on the left.
- Figure out how to distribute those atoms among the compounds on the right side.
- Then you can work backwards to find how many of the simpler $O$ compounds you have on the left side.
Ethanol (alcohol) $$C_2H_6O +\text{__}O_2 \rightarrow \text{__}CO_2 + \text{__}H_2O$$
$$\text{_1_}C_2H_6O +\color{blue}\text{_3_}\color{black}O_2 \rightarrow \color{blue}\text{_2_}\color{black}CO_2 + \color{blue}\text{_3_}\color{black}H_2O$$ 7 $O$, 2 $C$, and 6 $H$ on each side.
Coal $$ C + \text{__}O_2 \rightarrow \text{__}CO_2$$
$$ 1C + \color{blue} \text{_1_} \color{black} O_2 \rightarrow \color{blue}\text{_1_}\color{black}CO_2$$ That is, 1 Carbon atom, 2 Oxygen atoms on both sides. This is usually written without the ones, as: $ C + O_2 \rightarrow CO_2$
Methane (natural gas) $$CH_4 +\text{__}O_2 \rightarrow \text{__}CO_2 + \text{__}H_2O$$
$$\text{_1_}CH_4 +\color{blue}\text{_2_}O_2\color{black} \rightarrow \color{blue}\text{_1_}\color{black}CO_2 + \color{blue}\text{_2_}\color{black}H_2O$$ 4 $H$ and 4 $O$ and 1 $C$ on each side.
Ethane $$2C_2H_6 +\text{__}O_2 \rightarrow \text{__}CO_2 + \text{__}H_2O$$ Hint: Work out first how many molecules you need on the right to balance the $C$ and $H$. Then come back to the oxygen on the left.
The only molecule on the left with carbon or hydrogen is Ethane, $C_2H_6$, and we're starting with 2 of those. In one molecule of ethane there are 2 $C$ atoms and 6 $H$ atoms. So in two molecules there must be 4 $C$ atoms and 12 $H$ atoms. That's all the $C$ and $H$ on the left.
Switching to right side of the equation: the only molecule with any $C$ is $CO_2$ with one atom of $C$ per molecule. To balance the 4 $C$'s on the left we must have four molecules of $CO_2$. Similarly, 6 $H_2O$ molecules would have 12 $H$'s. So...
$$\text{_2_}C_2H_6 +
\text{_?_}O_2 \rightarrow \text{_4_}CO_2 + \text{_6_}H_2O$$
Now, we can figure out how many atoms of $O$ on the right. the 4 $CO_2$ molecules have $4\times 2=8$ $O's$. and the 6 $H_2O$ molecules have 6 $O$'s. So that's $6+8=14$ O's on the right.
Looking at the left side, we only have $O_2$ molecules, with 2 $O$'s each. So 7 molecules of $O_2$ will have 14 atoms on the left. The final, balanced equation is:
$$\text{2}C_2H_6 +\color{blue}
\text{7}\color{black}O_2 \rightarrow \color{blue}\text{4}\color{black}CO_2 + \color{blue}\text{6}\color{black}H_2O.$$
It would also be true to write:
$$\text{_1_}C_2H_6 +\frac 72 O_2 \rightarrow \text{_2_}CO_2 + \text{_3_}H_2O$$
This is fine as far as I'm concerned! Later, when we use this in combustion, you'll get the right answers when we calculate.
But some chemists want there to be *only integer* numbers in front of each molecule. To satisfy these folks, you can just multiply *all* the numbers of molecules by 2 (if you get any number with 2 in the denominator).
Chemical Energy
It takes energy (from the battery) to make the separation of water into hydrogen and oxygen reaction happen. The amount of energy depends on how many molecules you have. Sometimes this energy is put into the reaction equation explicitly like this: $$2H_2O_{\text{(l)}} +\Delta E \rightarrow 2H_\text{2 (g)} + O_\text{2 (g)}$$ where $\Delta E$=33.9 kcal / (gram of hydrogen). To put this number in context, see this table of "Heats of combustion" of some common hydrocarbons.
But the reverse reaction: $$ 2H_\text{2 (g)} + O_\text{2 (g)} \rightarrow 2H_2O_{\text{(l)}}+\Delta E $$ releases energy. The energy released, which is exactly 33.9 kcal / (gram of hydrogen), comes out (dramatically) as heat and light: An explosion.

Tom Gauld for New Scientist

More tragically,
this was the burning of the hydrogen-filled Hindenburg zeppelin in 1937
"Combustion" is the chemical term for what we commonly call "burning". It involves re-combining the atoms in molecules into new compounds that contain oxygen.
Some chemical bonds are "tighter" than others. When atoms are re-arranged in a chemical reaction, the new chemical bonds may take more or less energy to form than the old ones. $\Delta H$ is the difference in energy between the substances on the left and the right of a chemical equation and is also called "chemical energy".