Burning hydrocarbons

The last UN Secretary, Ban Ki-moon, said:
Energy is the golden thread that connects economic growth, social equity, and environmental sustainability".
Currently, most of *our* energy comes from combustion of fossil fuels

U.S. Energy Information Agency, U.S. Energy Facts
- Fossil fuels (oil, coal, natural gas) all result in $CO_2$ emissions.
- Nuclear energy: ~ 0 $CO_2$ emissions *.
- Renewables: ~ 0 $CO_2 emissions *.
(*) No $CO_2$ emissions during operation, but there are $CO_2$ emissions involved in manufacturing solar panels, or wind turbines, and in making concrete for dams and nuclear plants.
In the United States, CAFE - Corporate Average Fuel Economy standards were first regulated in 1978.
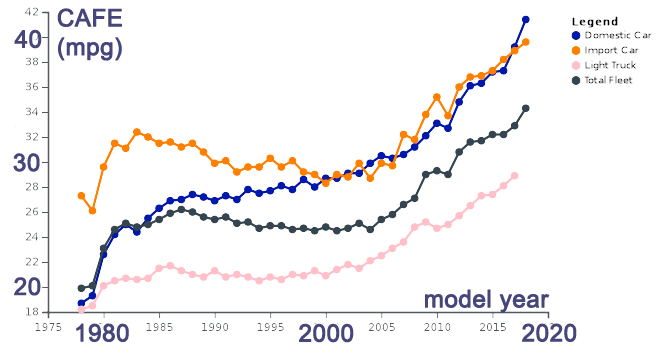
1973:
 Minnesota Public Radio
Minnesota Public Radio
Are there differences in emissions for the different fossil fuels?
Hydrocarbons and carbon-dioxide
"Hydrocarbons" contain hydrogen and/or carbon.
Burning hydrocarbons re-combine with oxygen to give a mix of $CO_2$ and $H_2O$.
Where does the oxygen come from? Why do they combine with oxygen instead of nitrogen?
How much energy do you get by burning? The heat of combustion of compound $X$ is the amount of energy released (in burning) per gram of $X$.
This is a lab we do in "climate change":
- Weigh a candle (it's mostly "paraffin"): Its initial mass is $m_i$.
- Light it. Put it under a beaker of water. Watch the temperature rise.
- The temperature of 1 gram of water will change by 1${}^o$ C for every 1 calorie of heat energy absorbed, so you can calculate how much heat the candle gives off: Call it $Q$ calories.
- Blow it out. Weigh the candle. It weighs less: $m_f$.
Let's look for patterns in the heats of combustion of different hydrocarbons:
| model | hydrocarbon | Energy per gram, Bonds |
|---|---|---|
 [GAS at standard temperature and pressure (STP) of 1 atm, 0${}^o$C.] |
hydrogen, $H_2$ | 26 Calories, $H-H$ |
 [GAS at STP] |
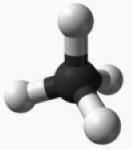
natural gas: methane $CH_4$ |
13. $C-H$ |
 [LIQUID at STP] |
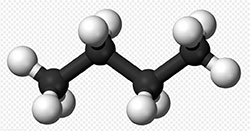
butane $C_4H_{10}$ | 11.8, $C-H$ and $C-C$ |
mixture of molecules with 5-12 carbons: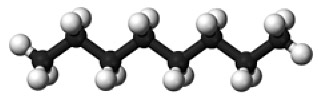 [LIQUID at STP] |
gasoline ~octane $C_8H_{18}$ | 10, $C-H$ and $C-C$ |
| mixture of hydrocarbons with 20-40 carbons per molecule [soft SOLID at STP] |
paraffin $C_{30}H_{62}$ |
10, $C-H$ and $C-C$ |
| Much more carbon, relative to other atoms. So, we'll approximate chemically as just pure carbon. [hard SOLID at STP] |
Coal: $C$ | 6, $C-C$. |
There are at least two patterns that you might notice here...
Why do we use gasoline for cars?
For the purposes of thinking about climate change, we should like to know not the energy / gram of the fuel, but rather the energy per gram of ... what?
This is the topic of the Combustion comparison page.
image credits
APS water, Robert Wallis/Panos Pictures, U.S. Energy Information Administration, EIA