Thermal energy

We have expressions for gravitational energy GravE=$mgh$ and so $$ mg\Delta h=\Delta\text{GraveE } $$
For kinetic energy, K=$\frac 12mv^2$ and so $$\frac 12mv_f^2-\frac 12mv_i^2=\Delta \text{KE}$$ where $v_f$ is the final speed and $v_i$ is the initial speed.
What about thermal energy? It turns out that a change in thermal energy is related to the change of temperature, $T$ like this: $$mc\Delta T = \Delta \text{ThermalE}.$$ where $c$ is the heat capacity of the substance you're working with. For example, for water, $c=1$ calorie / $1^o$ C /gm. (1 ml of water weighs 1 gram).
Problems
- Calculate how many calories it takes to heat 50 ml of water up by 3 ${}^o$C.
- Olive oil has a heat capacity of 0.47 cal / C / gm. How many calories does it take to heat up 50 ml of olive oil by 3 C?
- 100 ml of water cools from 38 C to 35 C. How many calories of thermal energy were lost?
Mixing water - final temperature
Your lab problem was to find the final temperature, $T$ when you mix $m_H$ ml of hot water at a temperature of $T_H$ with $m_C$ ml of cold water at a temperature of $T_C$. Using energy conservation, let's find the answer...
- If the hot water cools from $T_H$ down to $T$, how much energy does it lose?
- If the cold water heats up from $T_C$ up to $T$, how much energy does it gain?
- Set these energies equal,
- Solve for $T$
Mixing vs bags of water
What if, instead of mixing the water, you had the cold water in one plastic bag, and the hot water in another plastic bag, and then you set one down on top of the other. What would happen to the temperatures?
Is this because of energy conservation?
Where does our energy come from?
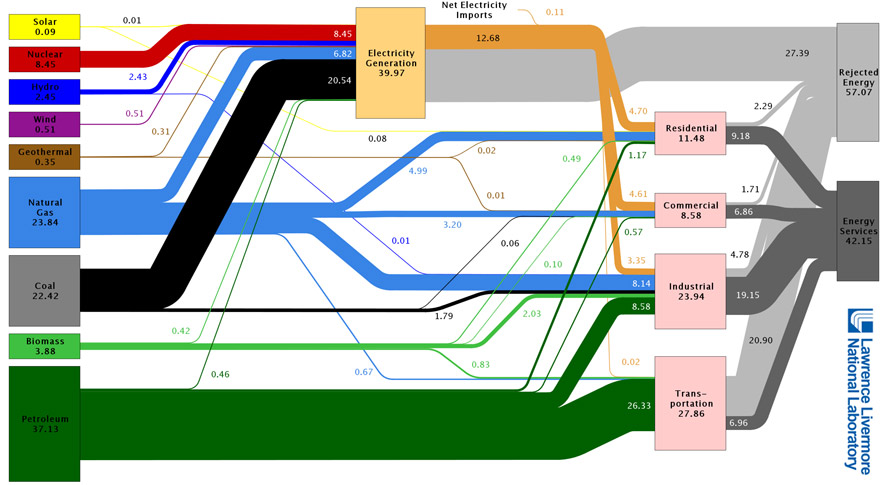
"Heat" Engines
A lot of modern engines work like this:
- Some form of input energy (not thermal),
- is converted to heat (thermal energy),
- which is then converted to mechanical energy.
 Many modern machines use heat to make some sort of mechanical motion
happen. In a gas engine...
Many modern machines use heat to make some sort of mechanical motion
happen. In a gas engine...
- The atoms in molecules re-arrange themselves into new molecules, and energy is released...as heat,
- The heat increases the temperature of the air in the cylinder.
- High temperature air has a much greater pressure than low temperature air, so the heated air pushes on the cylinder.
In a nuclear-powered  submarine,
submarine,
- The neutrons and protons in atomic nuclei re-arrange themselves into new nuclei, and energy is released...as heat,
- The heat is used to boil water into steam, which has a much higher pressure than the same amount of liquid water,
- the steam is forced through a turbine making a shaft turn...
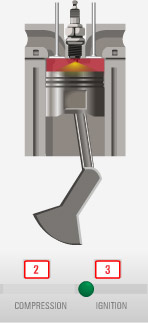
Steam engine
- Heat is generated, for example:
- Coal / wood / natural gas / etc is burned (ChemicalE $\rightarrow$ ThermalE) to generate heat, or
- A nuclear reaction happens (NuclearE $\rightarrow$ Thermal E) to generate heat,
- the heat is used to raise the temperature of water above the boiling point,
- generating high pressure steam.
You can use the steam to push a rod back and forth (howstuffworks.com) to power a steam locomotive,
or more commonly:
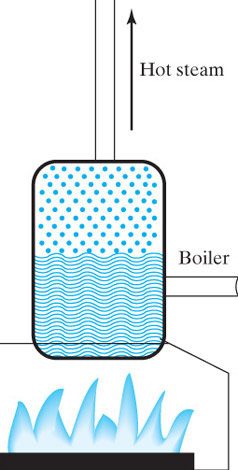
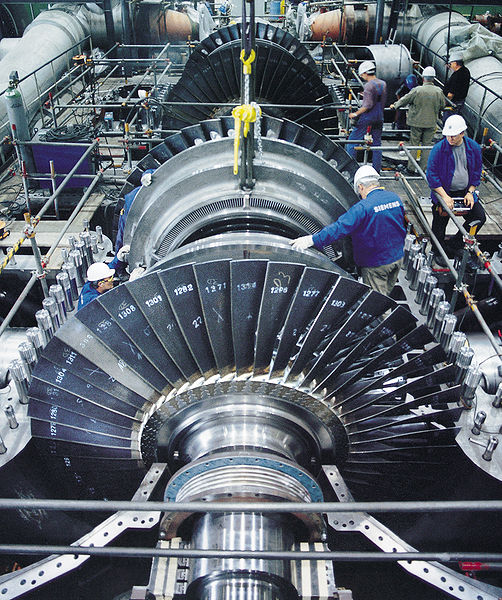 The expanding steam pushes against the blades of a turbine to make a shaft
turn,
The expanding steam pushes against the blades of a turbine to make a shaft
turn,
If you attach the shaft to magnets, and rotate them past electric coils you can cause a current to flow--*electricity*.
A heat engine is a device that uses thermal energy in some form, converting some of it into mechanical energy (KineticE) in a cyclic process.
Efficiency of heat engines
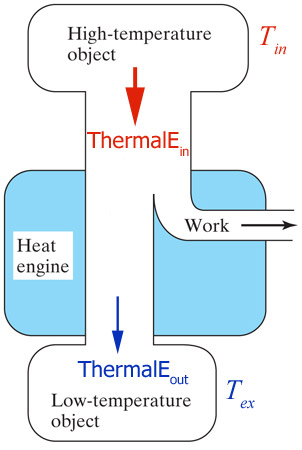 Just
like any other energy transformation, a portion of the input energy goes to...ThermalE.
Just
like any other energy transformation, a portion of the input energy goes to...ThermalE.
What's the efficiency of the cartoon heat engine shown?
energy efficiency = $\frac{W o r k_{out}}{ThermalE_{i n}}$
Example - A certain steam engine gets 50 kWh of heat from burning coal, and performs 12 kWh of mechanical work. What is its efficiency?
efficiency = 12 kWh / 50 kWh = 0.24 = 24%