Entropy?

...the pieces don't tend to fall back together.
Heat bags
...From last time:
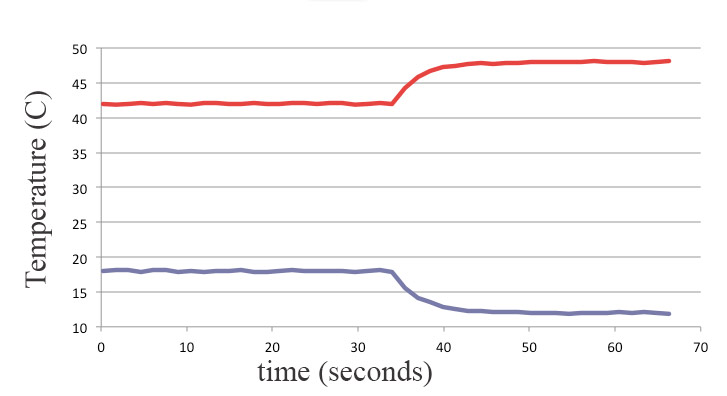
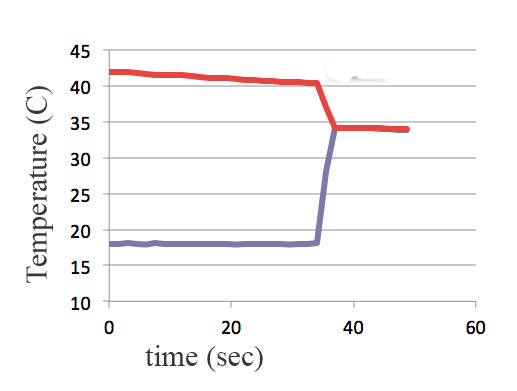
What if, instead of mixing the water, you had cold water in one plastic bag, and hot water in another plastic bag, and then you set one down on top of the other. Which of these Temperature($t$) graphs do you think really happens?
Which of these is compatible with Energy Conservation?
"For some reason" thermal energy flows spontaneously from a hot object to a cold object, but that reason is *not* energy conservation. (One way for heat to flow).
The name for that pattern or tendency is the Second Law of Thermodynamics. (One way flow of heat).
Second Law of Thermodynamics - in terms of heat
Thermal energy flows spontaneously from higher to lower temperature, but not from lower to higher temperature.
The First Law of Thermodynamics - Energy conservation: Energy can be converted from one form to another, but the total energy of any closed system is always conserved. (Thermal energy is one form of energy).
Incorporate answers to reading questions into class
One way flow...of time?
You hop into the middle of these two movies... Can you tell if the movie is being played forwards or "backwards"?
- swinging pendulums
- What about this one about a guy who realizes what a loyal friend his pillow is, after a breakup?
In which movie was there less friction in the systems being filmed?
even the one that you can tell is shot backwards doesn't violate any laws of physics that we know about so far:
Why *couldn't* a bunch of water molecules in a lake all of a sudden find themselves moving in the same direction, so as to eject the human swimmer??
And yet we know this rarely ~ never happens.
Friction
...is the conversion of energy $\to$ heat.
By "heat" we mean the kinetic energy of many molecules moving in all directions = "disorderly" energy.
The kinetic energy of a moving book represents *orderly* motion: all the molecules are moving together in the same direction, with the same speed.
So friction involves the conversion of "ordered" energy to "disordered" energy.
Another consequence of the 2nd law: It is "harder" to convert heat to ordered forms of energy, than vice versa.
Coin-flipping exercise
If you are fascinated by the meaning of entropy, read The Library of Babel--a short story by Jorge Luis Borges.
"Heat" Engines
A heat engine is a device that uses thermal energy in some form, converting some of it into mechanical energy (KineticE) in a cyclic process.
Efficiency of heat engines
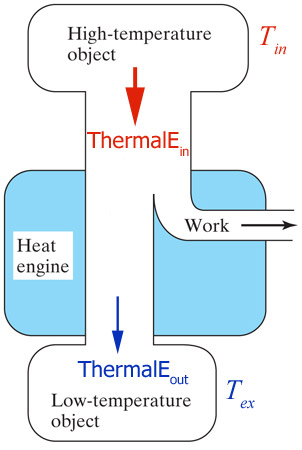 Just
like any other energy transformation, a portion of the input energy goes to...ThermalE.
Just
like any other energy transformation, a portion of the input energy goes to...ThermalE.
What's the efficiency of the cartoon heat engine shown?
energy efficiency = $\frac{W o r k_{out}}{ThermalE_{i n}}$
Example
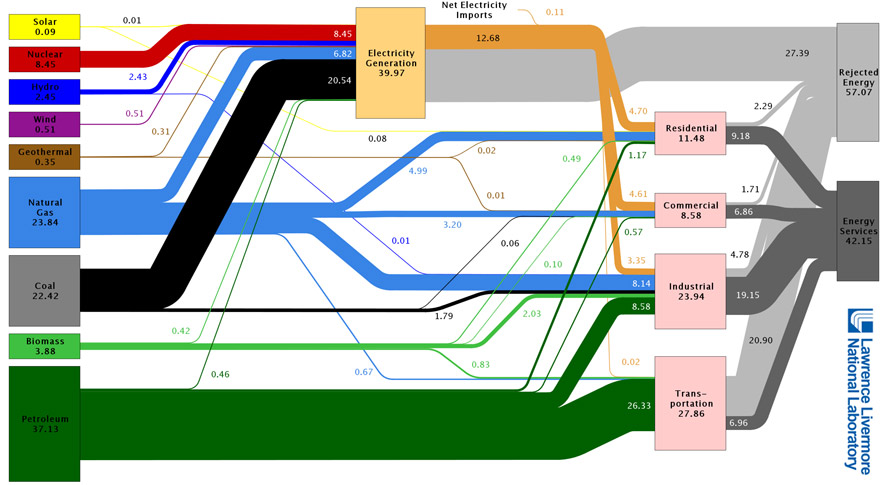
What is the efficiency of generating electricity from all energy sources in this country?
What is the efficiency of transportation in this country?