How does the sun heat earth?
And how does the greenhouse effect work?
Atomic picture of heat
Fundamental to what we'll be discussing is the idea that temperature is related to the speed of molecules.
Keep this simulation (PhET) in mind.
A demonstration of the difference between hot and cold water.
Neither beaker is moving: .mov or .wmv
Changing temperature BY CONTACT
The most familiar way to lose/gain thermal energy is by contact with something at a different temperature.
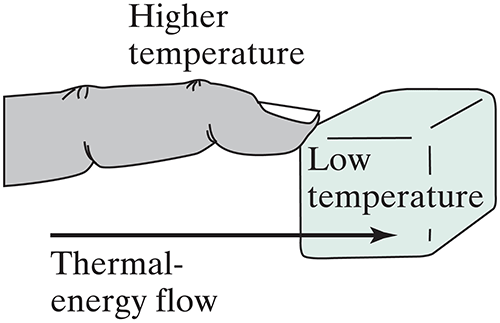
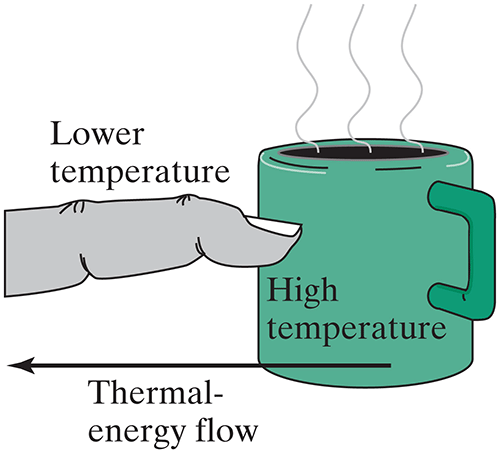
With someone close by: In terms of hot object (with fast moving molecules) and a cool object (with slow moving molecules) explain what happens after the two objects have been in contact for a while.
You fill an electric kettle with water, boil the water (100 C), and dump the water out (into a teapot). Now you leave the electric kettle turned off. You come back in an hour. Estimate the temperature of the electric kettle.
 But there is a second way to lose/gain thermal energy:
But there is a second way to lose/gain thermal energy:
What form of energy can you see coming from the bricks or from inside a hot kiln?
Remember that frequency ($f$), wavelength ($\lambda$), and the speed of (light) waves ($c$) are related by: $$\lambda=c/f,$$ So, high frequencies = short wavelengths (and vice-versa).
The spectrum of light given off by an object at temperature $T$:
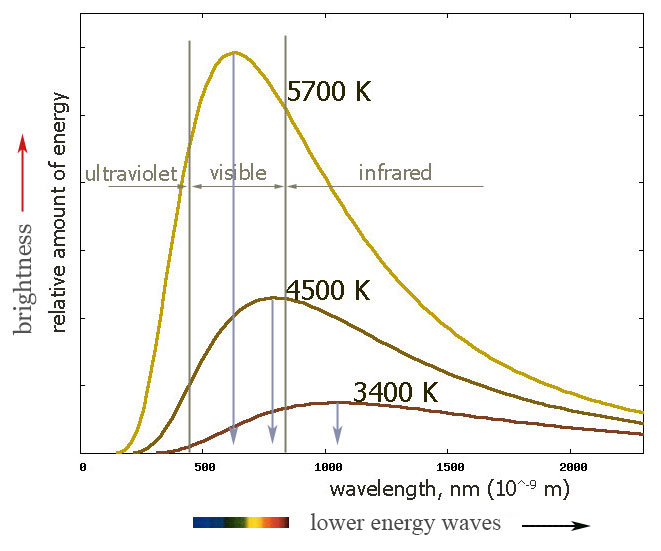
- is fairly independent of exactly what it's made of.
- It's a broad "spectrum" of different wavelengths (frequencies) of light.
- The peak wavelength shifts with temperature.
- At the temperature of our sun, ~5700K, there's a lot of light in the visible part of the spectrum! (Which is at least part of the reason our eyes have developed to be sensitive to "visible" light, more than IR or UV light.)
- A tungsten filament in an incandescent light bulb is running at up to 3300 K. (Tungsten melts around $3700 K$.)
This spectrum is called "blackbody radiation".
Changing temperature with light
We talk about the sun *heating* Earth, but Earth and sun are not in contact. 10-20 km above our atmosphere, there are almost no molecules. Space is a "vacuum".

It seems like the heat from the sun is related to the light. People (and others) take shelter from the light of the sun when it's hot outside.
This would not work if the only way you can get heated up is through contact with hot air.

Light can be absorbed, or transmitted, or reflected
- What color might an object be that absorbs a lot of light?
- What color might an object be that reflects a lot of light?
- What color might an object be that transmits a lot of light?
You leave a black chair and a white chair out in the sun on a day when the air is 90 F. Estimate the temperature of the black chair and the white chair when you come back an hour later.
Ye olde book-dropping picture...
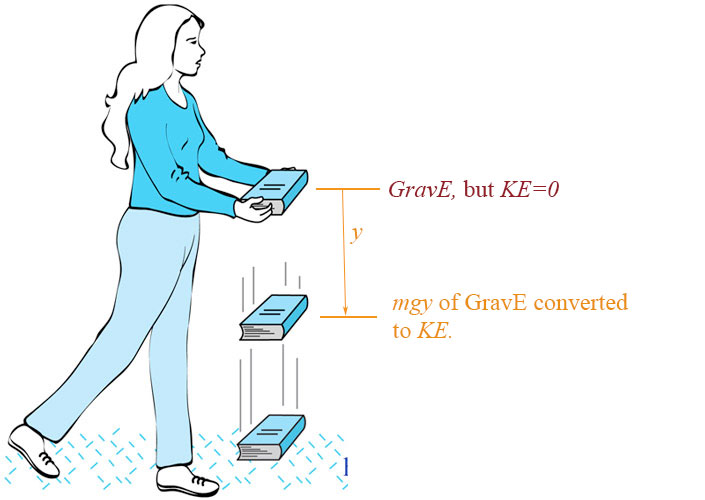
Light also has energy. Do you think more energy is left behind when light is reflected or absorbed.
Why aren't these birds beating their wings more?
In California, people are encouraged to have light-colored roofs to cut down on the energy needed for air conditioning. Why would having a light colored roof save on air conditioning?
You find yourself in California, and, looking up at the sky, you remark, rather nervously, that there are a number of hawks doing lazy circles in the sky. Are the hawks more likely to be above...
a) a large Wal-mart store with a roof painted white? or
b) a large, black asphalt parking lot?
Where does light come from?
Whenever you shake (accelerate) some charged particles you send out an electric disturbance, a wave. These are called electro-magnetic waves. Light is one kind of electromagnetic wave.
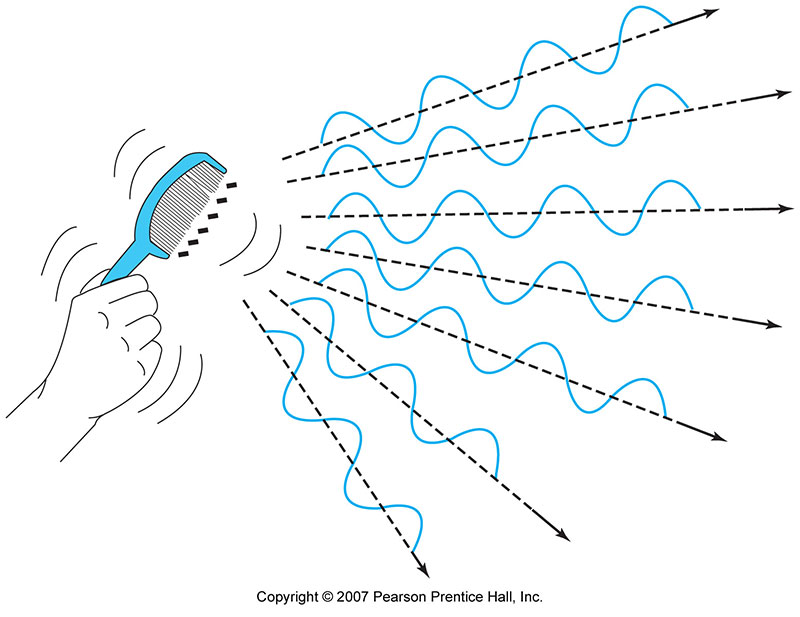
Waves in air ("sound") or waves on a string are disturbances in a "medium" that move through the medium. (The air, or the string).
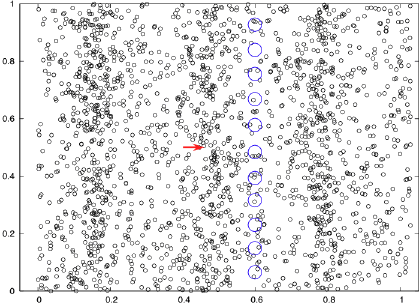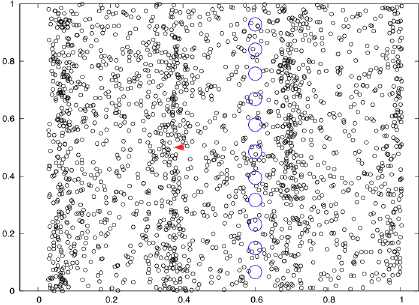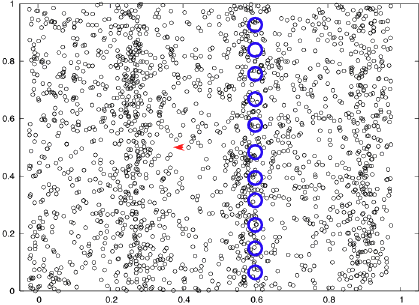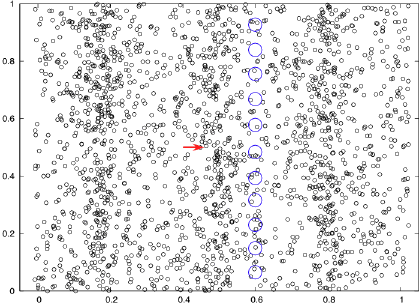
What is required for light to go from place to place? What does it travel through? What (if any) "medium" is requred for light to move through?
Talking about waves

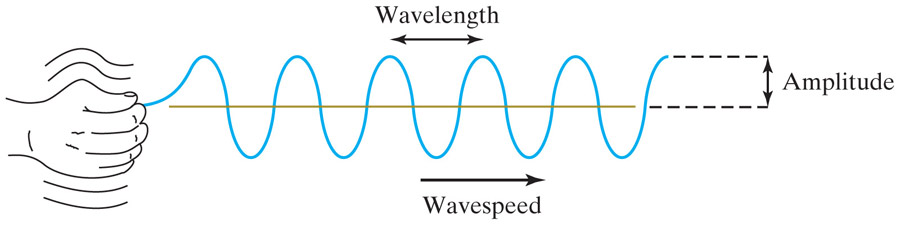
Wavelength, $\lambda$ = distance between crests in [m] (or [m / (wave)])
Wavespeed, $c$ - units [m/s]
Amplitude = maximum distance away from 'undisturbed' (yellow line) - units [m]
Frequency, $f$ =
# of waves passing a point in a time interval: [(waves)/sec] or [(cycles)/sec] =
[Herz] =[Hz]
Or, how 'fast' your hand shakes as it sends out waves on the slinky.
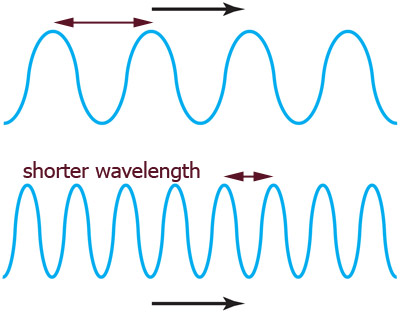 When you shake faster the wave peaks get closer
When you shake faster the wave peaks get closer
1.) Shaking *really fast* results in waves with (a) a low frequency, or (b) a high frequency?
2.) Shaking *really fast* results in waves with (a) a small wavelength, or
(b) a long wavelength?
It turns out that speed, $c$, wavelength, $\lambda$, and frequency $f$, are related by: $$\lambda = \frac{c}{f}$$ More commonly written as $c=\lambda f$.
Some common wave speeds:
- Sound ~ 760 mph= 340 m / s
- Light ~ $3*10^8$ m/s = 300,000,000 m / s
Temperature and E-M waves
We know that
- Temperature causes things to move and "shake" faster.
- Something that shakes faster has a higher frequency.
- When charges shake, they give off electro-magnetic waves.
So, there is a connection between temperature and frequency of light waves given off.

Based on your experience with hot objects:
1.)Do you think the parts glowing (a) orange or (b) yellow in this picture are
hotter [shaking faster]?
So, do you think (a) orange or (b) yellow has the higher frequency?
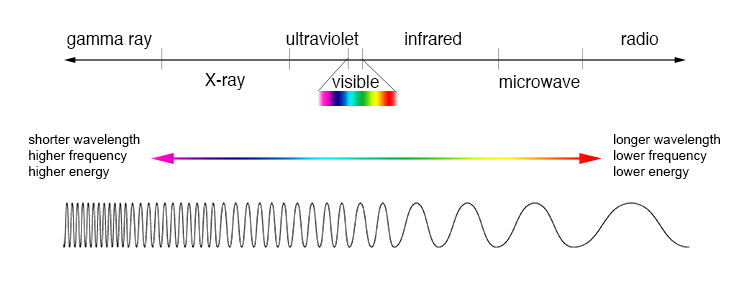
Here is a diagram of the Electromagnetic spectrum by frequency and by wavelength:
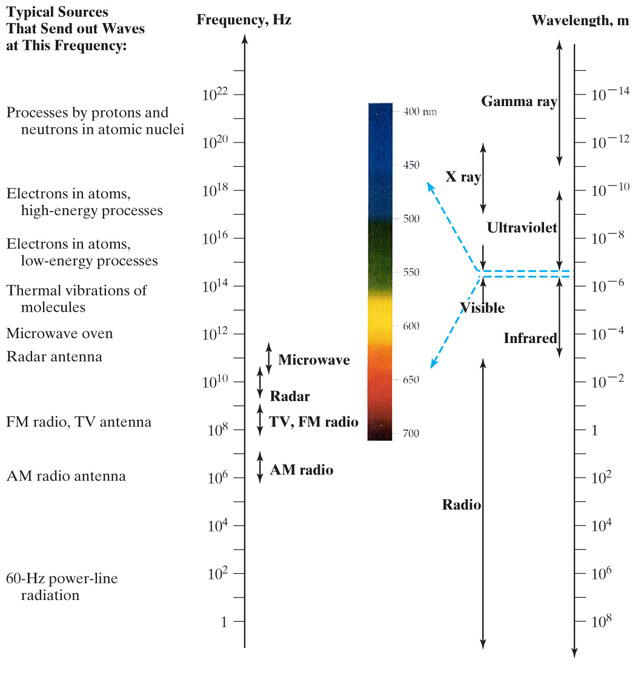
Our sun has a surface temperature of ~5400 C ~5700 K, and gives off a light of light in the "visible light" part of the spectrum.
We are warm too. Do we glow? If so, what part of the electromagnetic spectrum would it be, given our temperature of about 40 C?
We can't see "ultraviolet" light, but some insects can.
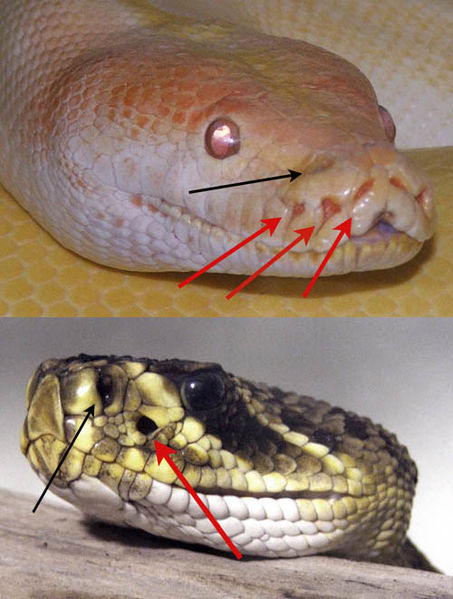 We can't see "infrared" (IR) light, but some snakes can. [They have regular eyes as well as "pit organs" that are sensitive to IR light.]
We can't see "infrared" (IR) light, but some snakes can. [They have regular eyes as well as "pit organs" that are sensitive to IR light.]
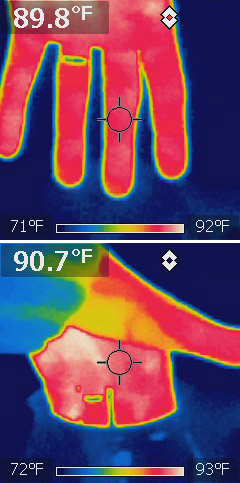
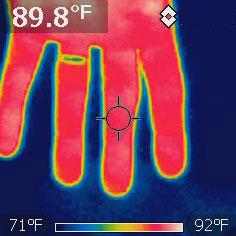 An infrared camera has an IR sensor, and then a microprocessor translates
the IR image into shades of visible light for us to look at.
An infrared camera has an IR sensor, and then a microprocessor translates
the IR image into shades of visible light for us to look at.
The Greenhouse effect
Let's design an "energy trap" for visible light!
When light strikes "stuff"
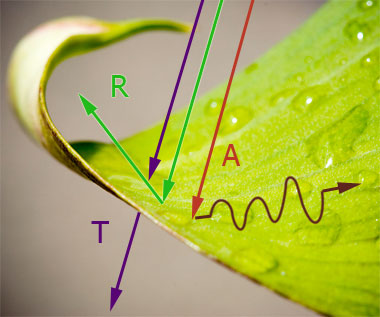 Three kinds of things can happen when light strikes something, depending on frequency and the material:
Three kinds of things can happen when light strikes something, depending on frequency and the material:
- Transmission: the light can pass right through (windows).
- Reflection (scattering): the light "bounces" off (white surface), or
- Absorption (friction): the light comes to a stop, and its energy *heats* the object up.
Sometimes that transmission absorption depends on the wavelength.
1.)Based on our in-class demonstration with the IR camera... Is glass transparent to IR radiation or not?
2.) Based on your own experience, is glass transparent to visible light or not?
So, imagine that we put something *black* that absorbs light inside a glass container:
- Visible light shines *through* the glass. (Energy gets in)
- Some of that light is absorbed by the black object. (Transformation of visible light to heat).
- The black object gives off IR light. (Radiative cooling)
- But the glass is *not* transparent to IR, so the IR light does not exit the glass container.
So, energy from light has entered the container, but can't get out. And therefore things in the container *heat up*.

a.) What would happen to the temperature inside if there were no black object inside to absorb the light?
b.) Even with dark objects inside, what would happen to the temperature inside if we made the container out of different material that *is* transparent to IR as well as visible light?
The Greenhouse Effect
Now we have practically told the story of the Greenhouse Effect:
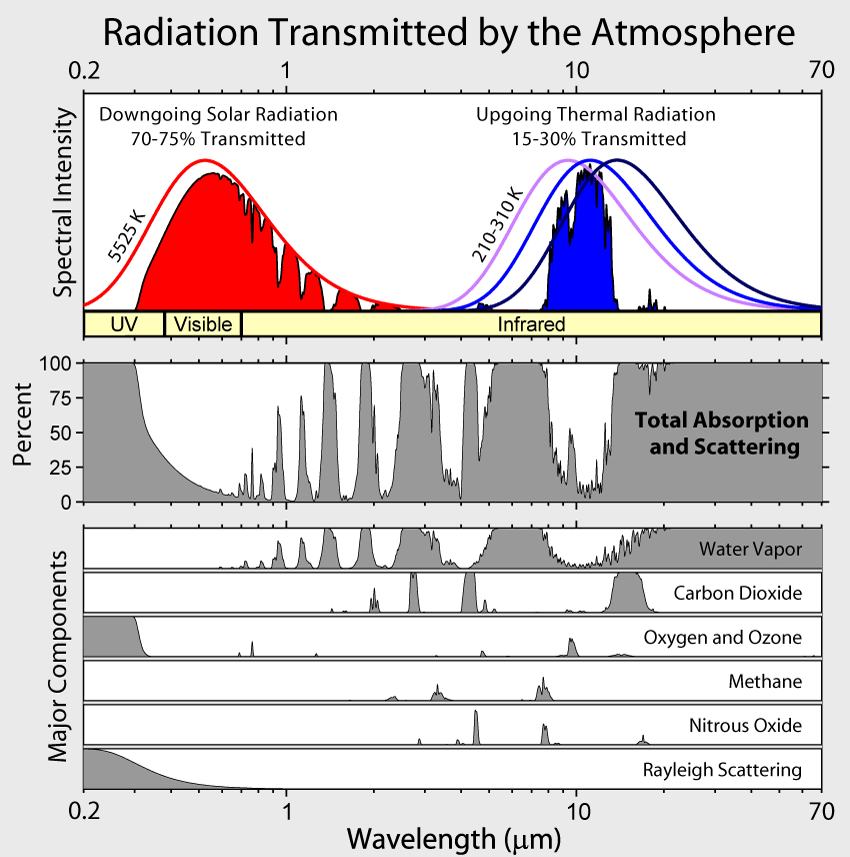
- Light from the sun in the visible region mostly passes freely through Earth's atmosphere.
- It strikes "stuff" on Earth, heating it up.
- The stuff radiates energy in the IR.
- But the atmosphere is not so transparent to IR radiation.
As a result Earth stays warmer than it would if there were no greenhouse gases. This is where gratitude kicks in:
Thank God!
Scientists estimate (NASA) that the "natural greenhouse effect" is the reason why the average temperature on Earth is about 59F.

<
If there were no greenhouse gases in Earth's atmosphere, Earth's average temperature (NASA-GISS) would be closer to 0F.

But, "anthropogenic warming" is the *additional* warming caused by humans dumping additional amounts of long-lasting greenhouse gases into our atmosphere.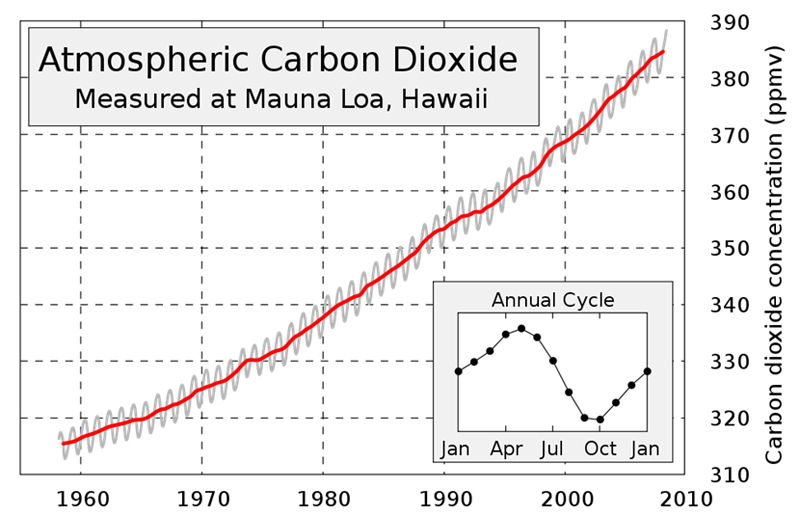
Now, one of the biggest challenges of our generation...
Image credits
Insolation map: NREL
Phil G, D Searls, Flickr user Zen, Wikipedia user Kendrick7 and S.n., TJFlex, Alex C, Rick Tree
$CO_2$, $H_2O$, $CH_4$ and other greenhouse gases absorb (some) light in the infra-red, instead of transmitting it.
Other gases like $N_2$, $O_2$, $Ne$, $Ar$ are transparent to IR light.