GHGs and GWPs
We can compare other GHGs to $CO_2$ in terms of their "Global Warming Potential" (GWP):
- The GWP of $CO_2$ is, by definition, 1.
- Other GHGs may have more or less "Warming Potential".
- But the impact of other GHGs also depends on how long they remain in the atmosphere.
- Methane has a GWP${}_{100}$ of 27 to 30 over a period of 100 years. Methane typically stays in the atmosphere for only about a decade. But it's "insulation" or "warming potential" is much higher than $CO_2$.
- This means that 1 unit (?) of methane emitted today would warm the atmosphere over the next 100 years (GWP${}_\text{100}$) as much as 27-30 units (??? what are the units) of $CO_2$ emitted today.
Read that EPA report on GWPs and/or their site about GHG emissions.
- Are GWPs calculated on a per-molecule or per-weight basis?
- What other gases have high GWPs? (and how high?)
- Where do those gases come from? (Why are they in the atmosphere?)
- We worry about $CO_2$ more than some of these other gases, even though they have much higher GWPs. Why?
Radiative Forcing
...A way to compare the immediate warming potentials.
"Radiative forcing" is a measure of how much of the solar energy which Earth receives actually stays in the atmosphere because of a particular gas.
Units of RF are energy (watts) per square meter. Usually what is depicted is the *additional* energy retained by the atmosphere relative to ... in this case pre-industrial levels.
- In pre-industrial times, was there any carbon-dioxide in the atmosphere?
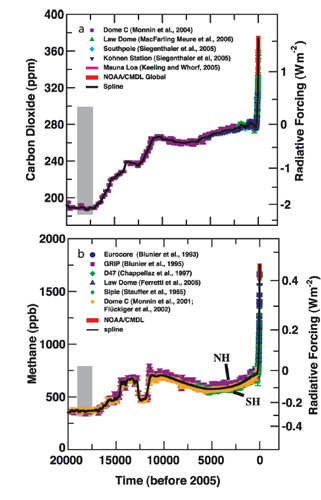
The gray bars indicate natural ranges in last 20,000 years.
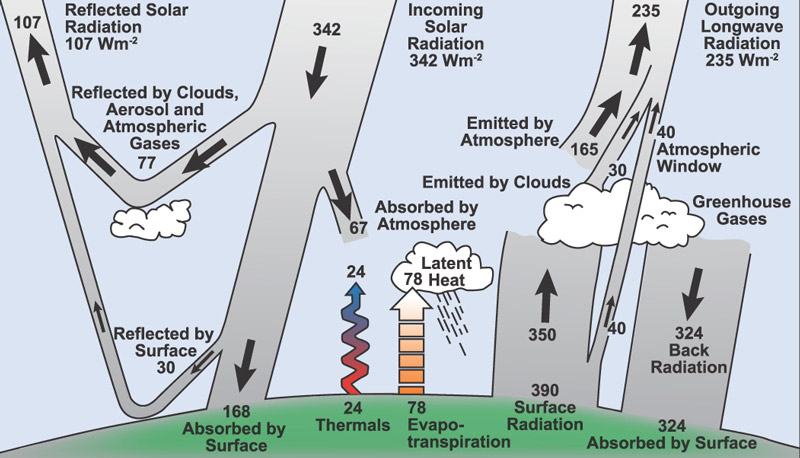
On average, each square meter of surface area in Indiana receives 150 Joules of solar energy each second, or 150 "Watts" per square meter. (1 Watt = 1 Joule / second).
Here's a schematic of all kinds of energy flows for
sunlight energy:
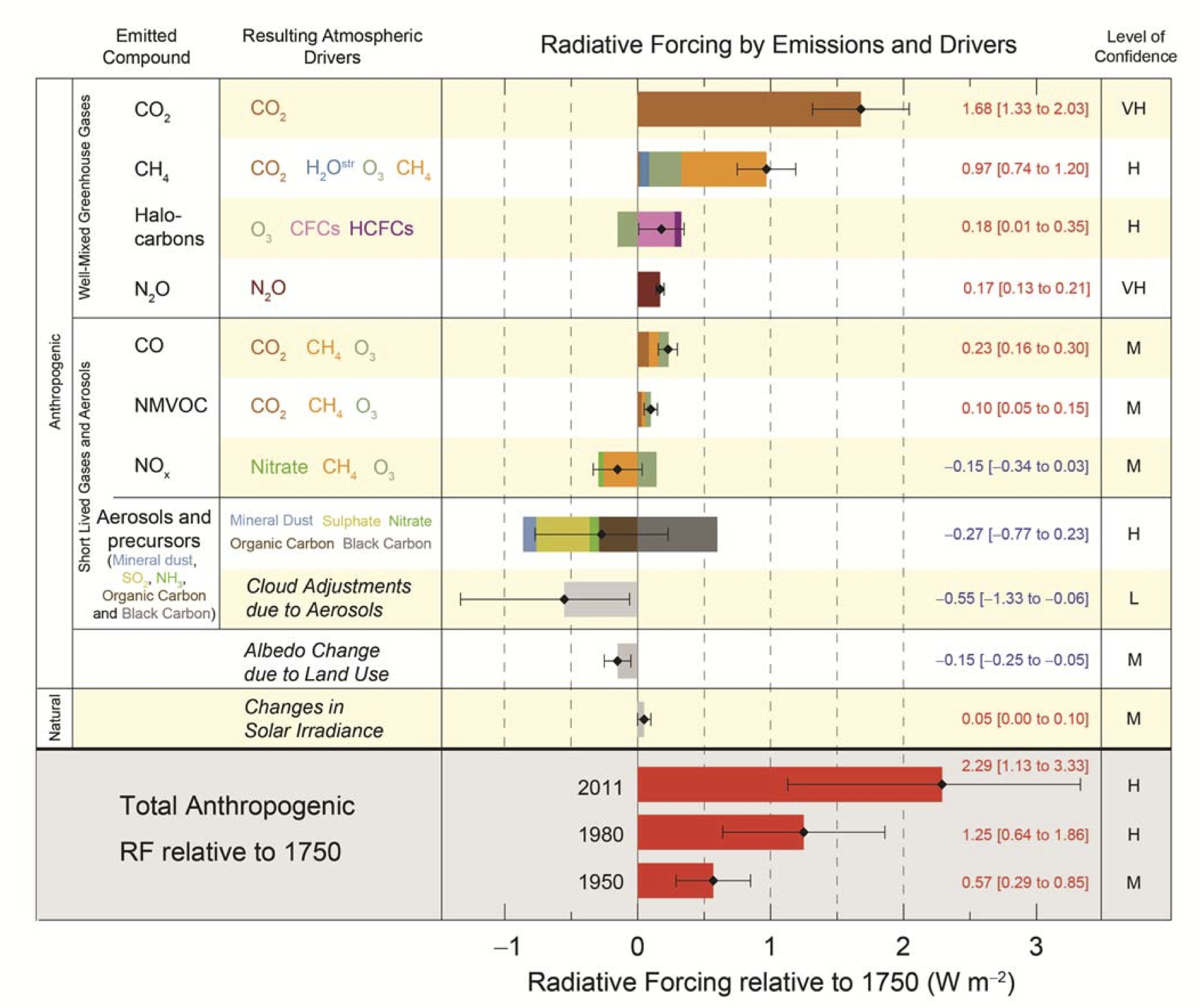
Here is a graph from the IPCC AR-5 report comparing GHG and other warming influences in terms of their "Radiative Forcing" (RF) relative to Earth's conditions in the year 1750 ("pre-industrial")
| Compound | GWP${}_{100}$ per ton | Source |
|---|---|---|
| $CO_2$ | 1 | |
| $CH_4$ methane | 27-30 | |
| CFCs | 1000 - 15,000 | |
| HFCs, HCFCs | 50 - 3,000 | |
| $N_2O$ | 265 | |
| $SO_2$ & aerosols | various |
Test study questions
- What are some common GHGs in Earth's atmosphere?
- What is "radiative forcing", and what units does it have?
- What are the sources of HFCs in Earth's atmosphere, and what could be done about them?
- $CO_2$ has a GWP of 1 and $CH_4$ has a GWP o 27-30. So why does $CO_2$ have a bigger impact on climate warming than $CH_4$?
Flaring (burning) natural gas
 There's a gas flare burning on the US 31 bypass near Elkhart, at the county landfill.
There's a gas flare burning on the US 31 bypass near Elkhart, at the county landfill.
- Bacteria decomposing organic waste in an anaerobic (no $O_2$) environment give off methane.
- There was a project to use some of this natural gas to heat the nearby prison.
- The rest is "flared" or burned off.
- When methane is burned, $CO_2$ is produced! $$1CH_4+2O_2\rightarrow1CO_2+2H_2O$$
Combustion generates carbon-dioxide. But which is worse for the environment... releasing the methane or burning the methane and producing carbon-dioxide?
- In the chemical reaction (above) that occurs for burning methane, $CH_4$ write the total molecular weights of methane and carbon dioxide involved in the reaction. The ratio of these two numbers is the weight ratio of methane to carbon dioxide in the this reaction.
- Calculate this number: To produce 1 ton of $CO_2$, how many tons of methane must you burn?
- The GWP${}_{100}$ of methane is 27-30 / ton. Call it 28 / ton. The GWP${}_{100}$ of $CO_2$ is (by definition) 1 / ton.
- Figure out the total GWP of the number of tons of methane that you calculated above: [total GWP is GWP/ton * tons of the gas] and the total GWP of your 1 ton of $CO_2$ (You should be able to calculate this number easily without resorting to a calculator!!)
- Is it better to burn the methane and turn it into carbon-dioxide, or is it better to just emit the methane without burning it? (Whichever you choose...How many times better?)
The net impact of a gas is its weight times its GWP.
The chemical reaction for burning methane is $$1CH_4+2O_2\rightarrow1CO_2+2H_2O$$ So there is on mole of $CO_2$ produced when one mole of $CH_4$ is burned. Calculate the molecular weights (M.W.) of these moles:
- M.W. of $CH_4$=12+4*1=16 grams/mole
- M.W. of $CO_2$=12+2*(16)=44 grams/mole
- (unburned) methane: Multiply the number of kg by methane's GWP: 4 kg*28=112
- $CO_2$ produced: Multiply the number of kg of $CO_2$ by it's GWP: 11 kg*1=11.
Bottom line: This means that expelling unburned methane will cause (112/11)~10 times as much warming as burning the methane and producing carbon dioxide.
Methane leaks
On the combustion worksheet you calculated that burning coal (0.56 g of $CO_2$ per Calorie of heat) results in higher $CO_2$ emissions than burning methane (0.21 g per Cal), Coal emits 0.56/0.21=2.7... almost 3 times as much $CO_2$ for the same amount of heat.
Natural gas has been touted as a cleaner fuel. But this relative advantage can be undone if too much of the natural gas leaks into the atmosphere.
 Both methane and carbon-dioxide readily transmit visible light--they are both invisible to the naked eye. But infrared cameras can 'see' methane.
Both methane and carbon-dioxide readily transmit visible light--they are both invisible to the naked eye. But infrared cameras can 'see' methane.
And satellites have been launched and more are in the planning stage which can detect large methane plumes from space.

 In the news: Because of the war in Ukraine, Russia has cut off natural gas exports to Europe. The U.S. has stepped up its exports of liquified natural gas (LNG). But a recent analysis concluded that Europe's emissions would be lower if it produced electricity by burning coal instead of importing LNG[!]
In the news: Because of the war in Ukraine, Russia has cut off natural gas exports to Europe. The U.S. has stepped up its exports of liquified natural gas (LNG). But a recent analysis concluded that Europe's emissions would be lower if it produced electricity by burning coal instead of importing LNG[!]
Harking back to the Paris agreement and those voluntary emissions limits, emissions from LNG and any other energy exports do not count towards the exporting country's emissions.